3DEXPERIENCE ENOVIA Role
Medical Device Engineer (DAR)
Creates a centralized, secure, digital repository between all global teams of all the data required to manufacture and test regulated products
Get Started on 3DEXPERIENCE.
- Accelerate product introductions, improve collaboration with outsourced partners and suppliers, and achieve compliance with confidence
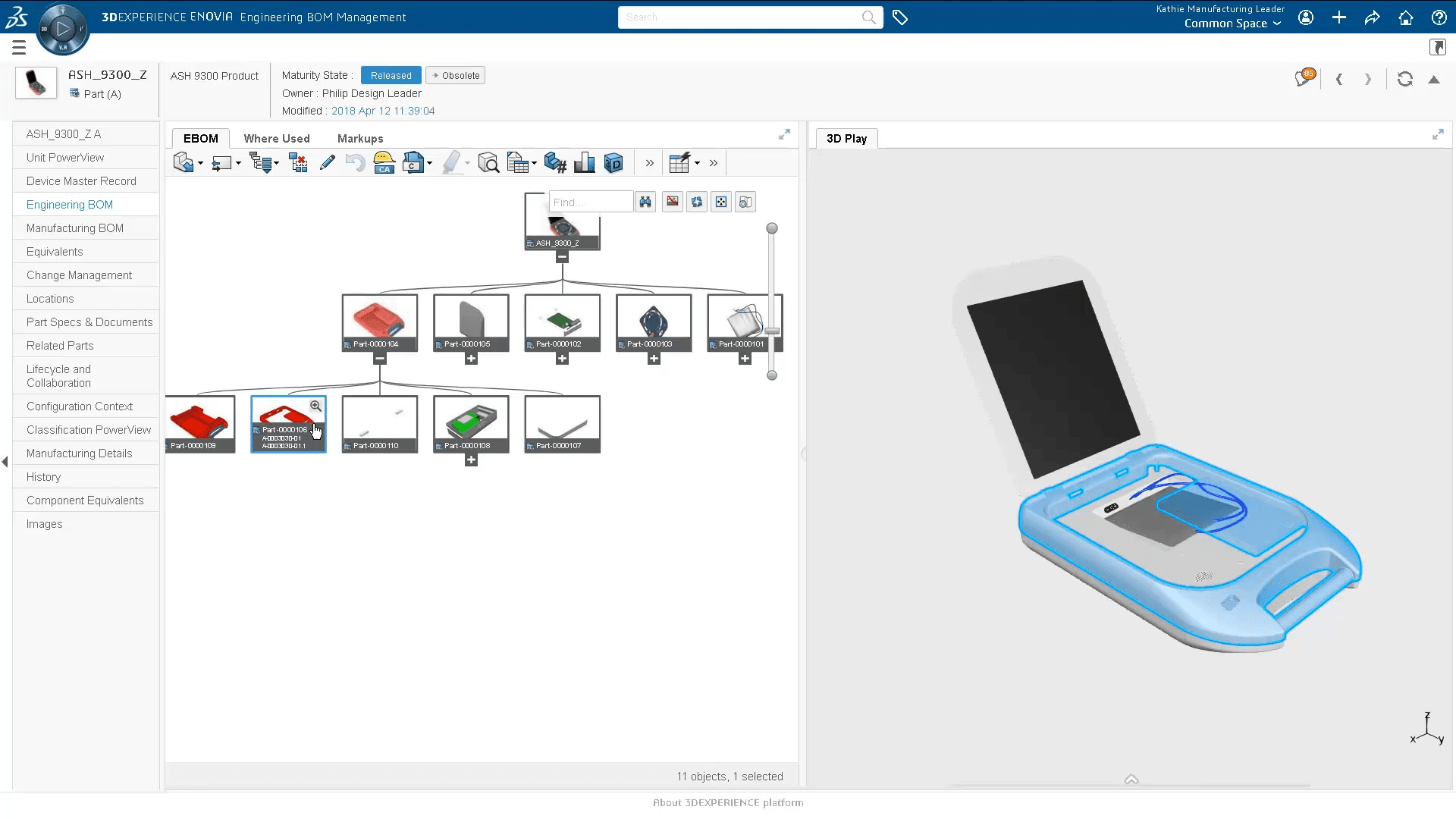
- Manage all design specifications, procedures, and instructions required to manufacture each type of finished product
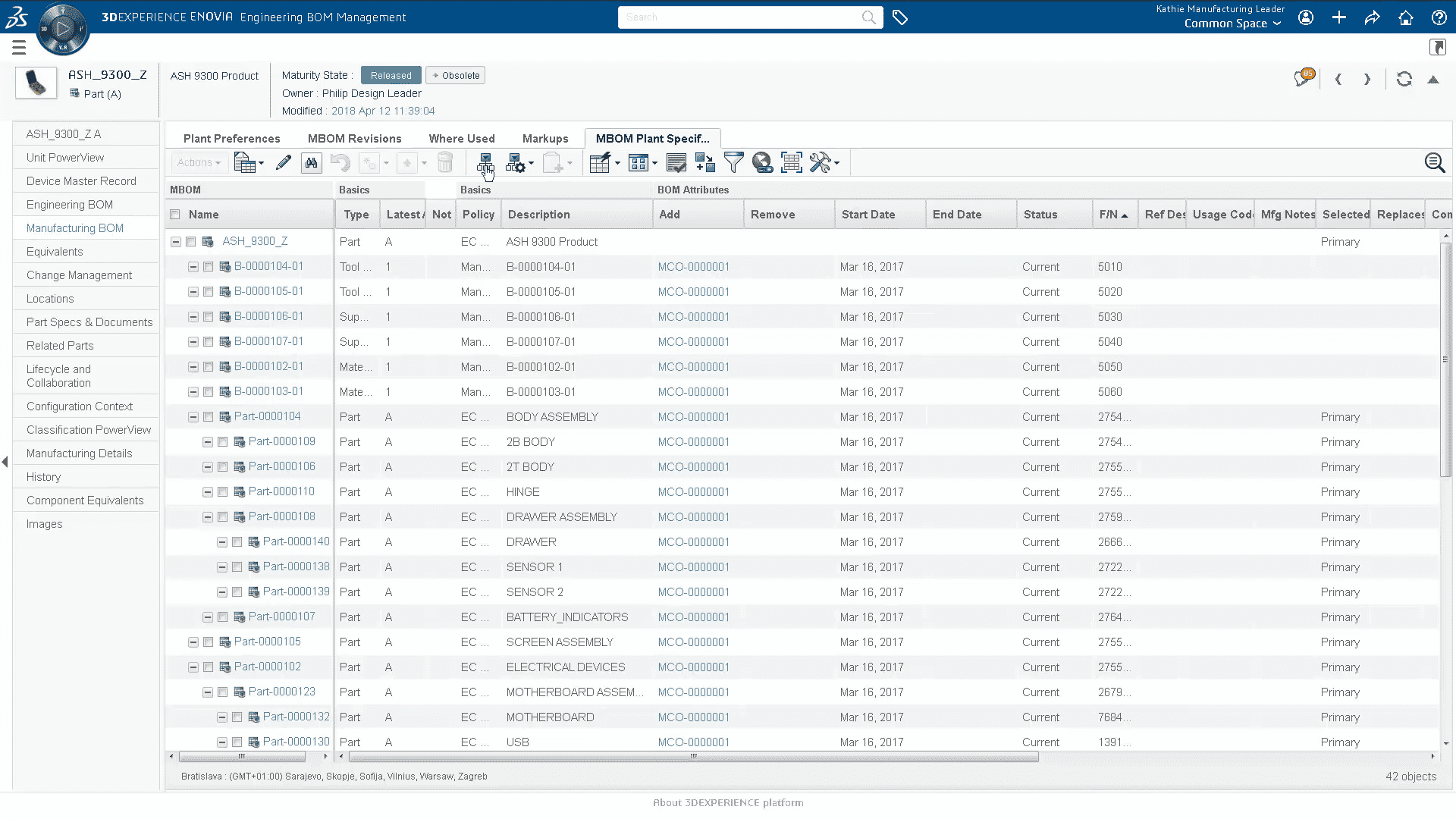
- Audited access to and download of all Device Master Record documents
- Plant specific “finish device” Device Master Record with effectivity filtering
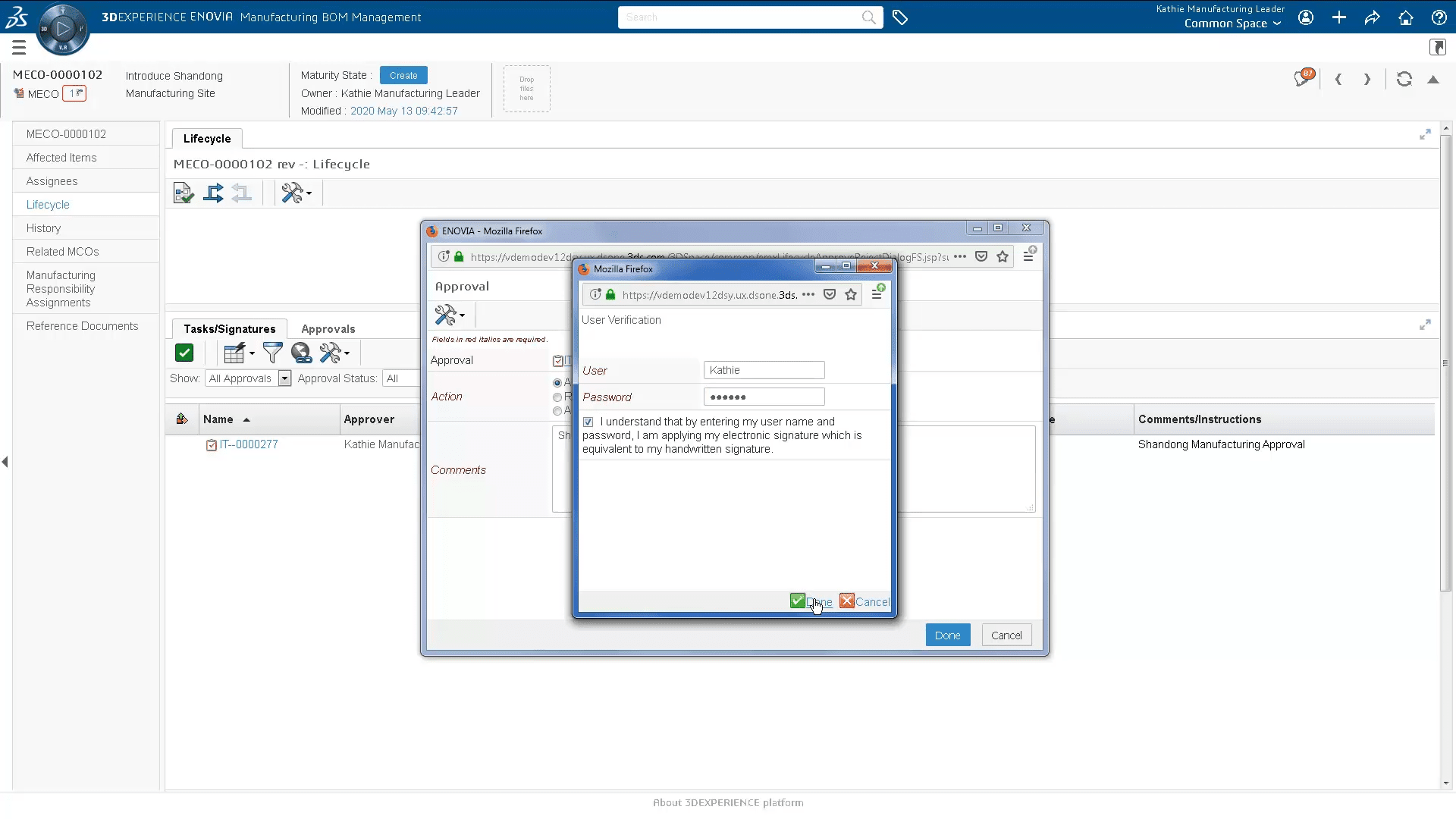
- Facilitate change to Device Master Record documentation
- Collaboration between engineering, quality, and manufacturing teams to share product information, exchange ideas and implement changes.
2 Included Apps

Device Master Record Management
Change Governance
3DEXPERIENCE Learning and Support
3DEXPERIENCE Learning Resources
3DEXPERIENCE is powerful and transformative software, so learning to make the most of it isn’t trivial. Thankfully, Dassault Systèmes and CATI provide plenty of resources on the path to proficiency:
- Official and custom-tailored classroom training
- Online training courses on the Learning Space
- CATI’s quick-response technical support
- Extensive official documentation
- Dassault Systèmes Knowledge Base of supplemental materials, technical articles, and support tickets
- CATI simulation mentorship programs
- CATI consultative methodology development
Why 3DEXPERIENCE with CATI?
Computer Aided Technology (CATI) has been implementing solutions on the 3DEXPERIENCE Platform since 2012. No one has more experience when it comes to 3DEXPERIENCE Platform solutions, especially when it comes to implementing the platform with SOLIDWORKS or CATIA.
Watch this video to learn more about why companies choose to partner with CATI for 3DEXPERIENCE solutions and get started today.
Get Started with 3DEXPERIENCE from CATI.
We’re ready to help figure out the right product development solution plan for you. Contact us today to get started with a quote, a trial, a demo, or just ask questions.